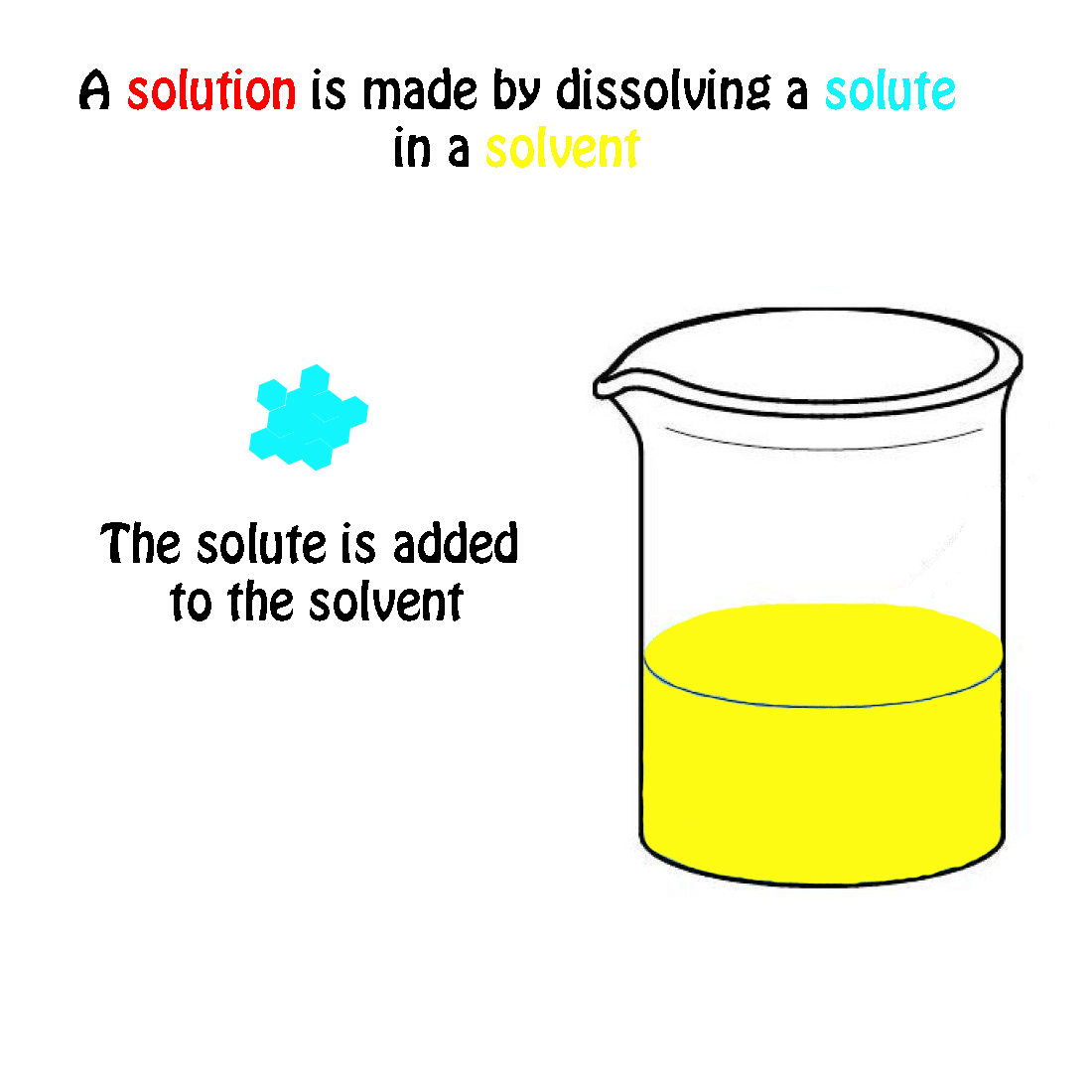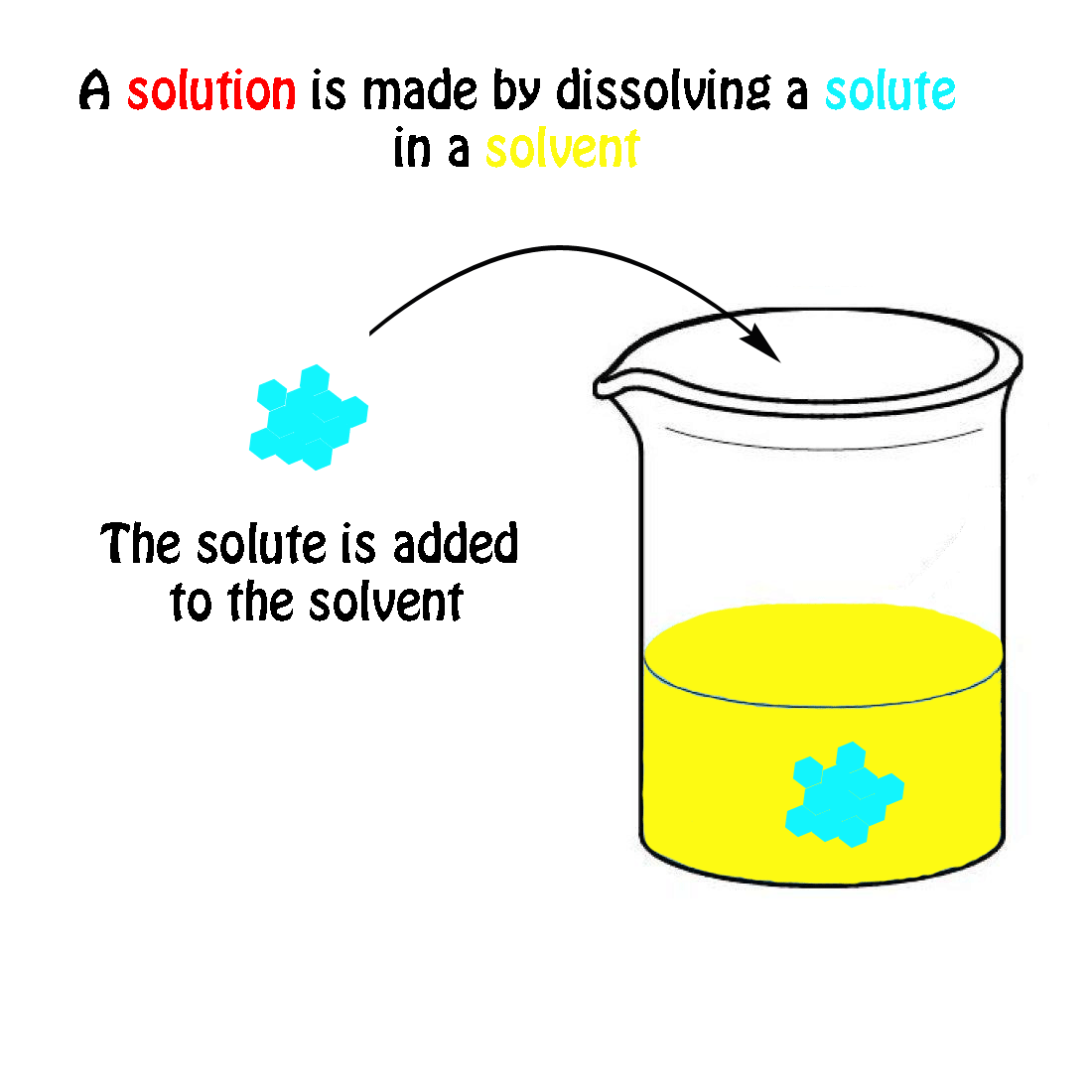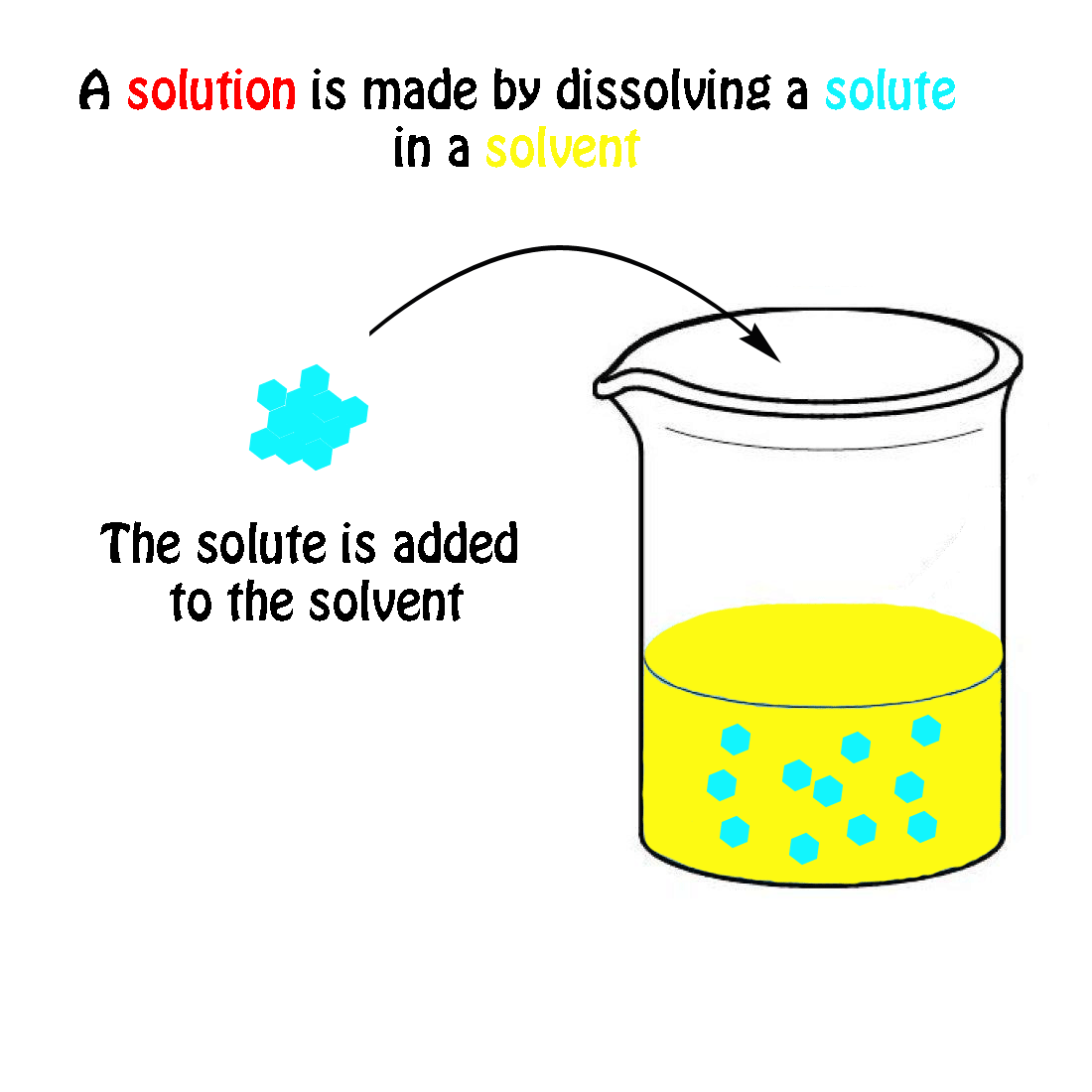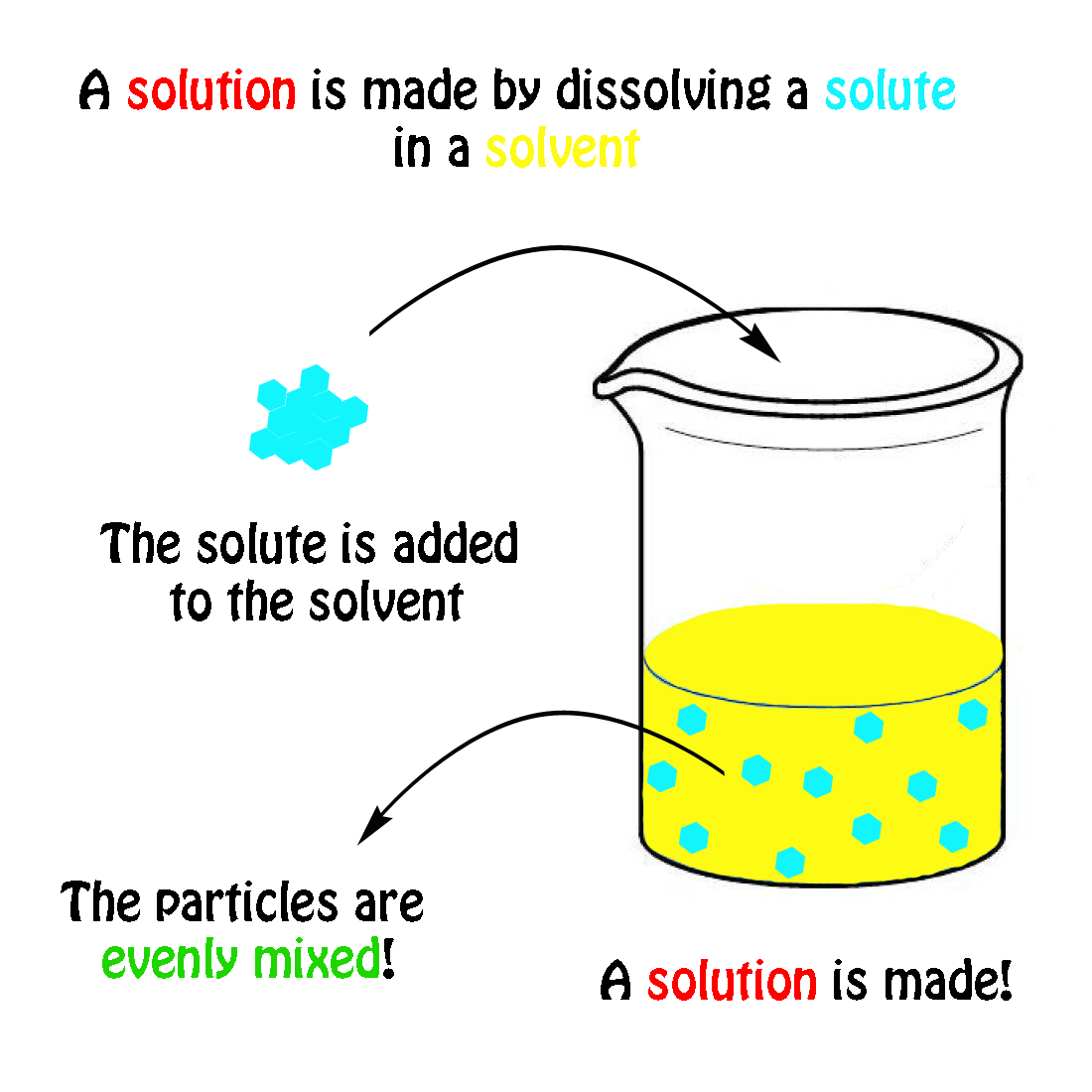
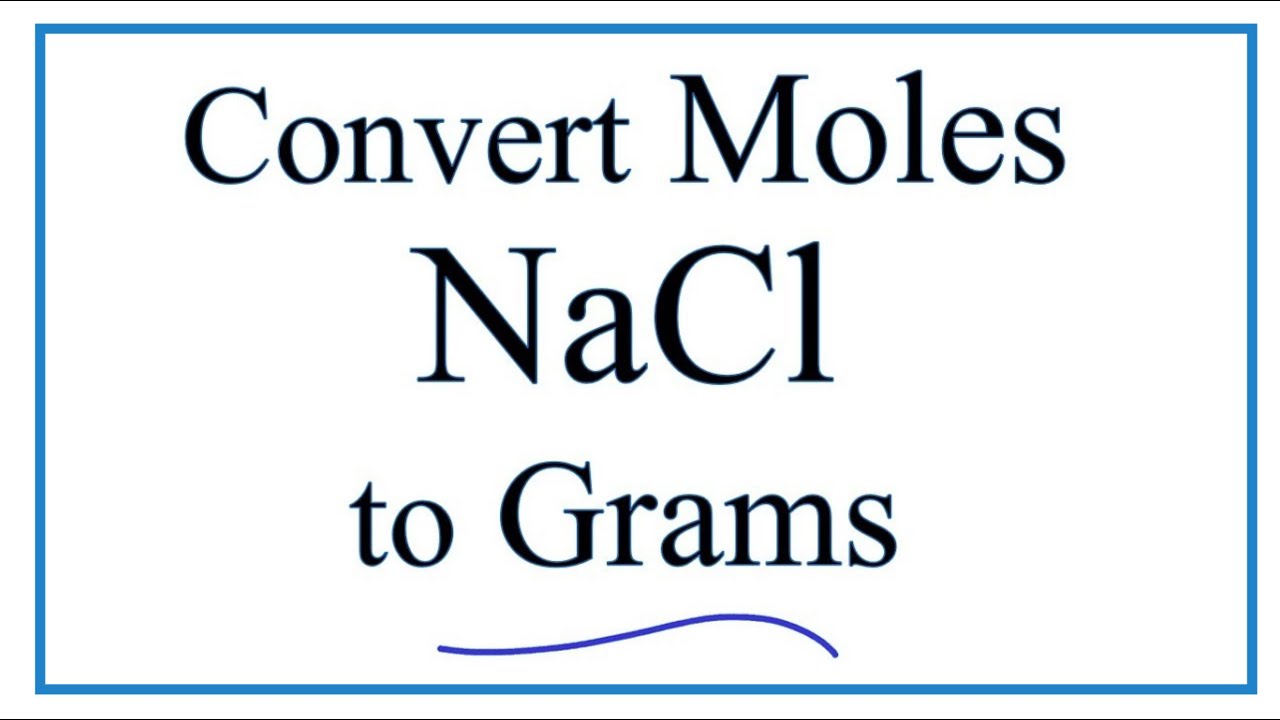
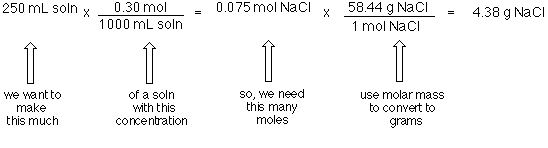The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download
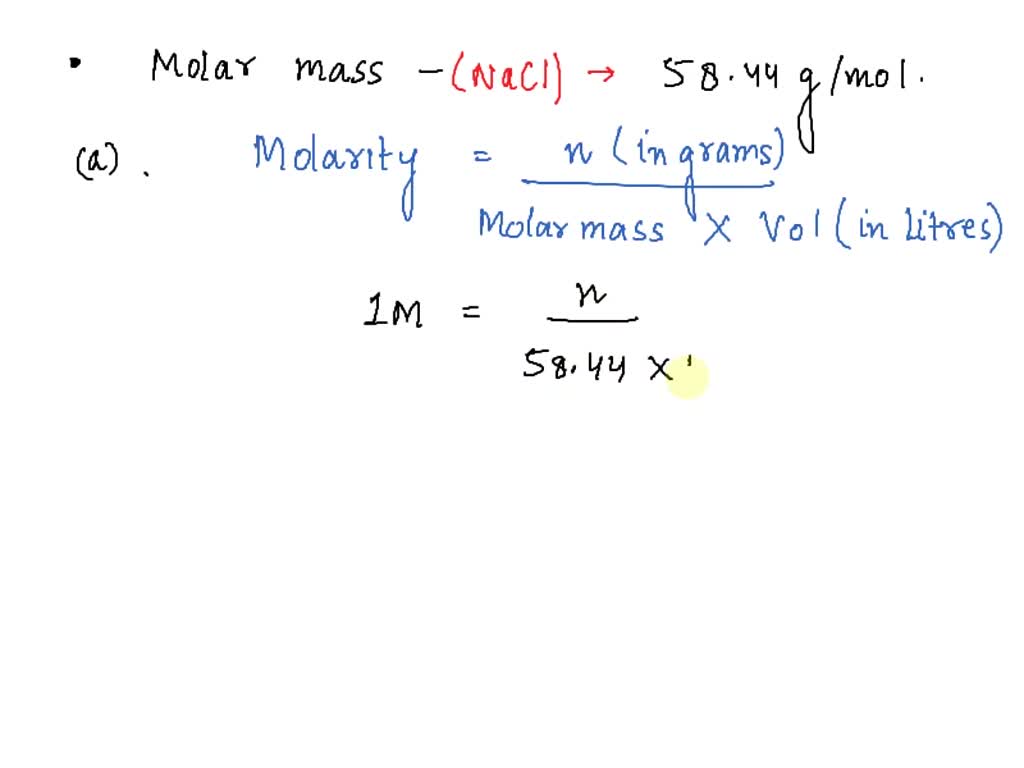
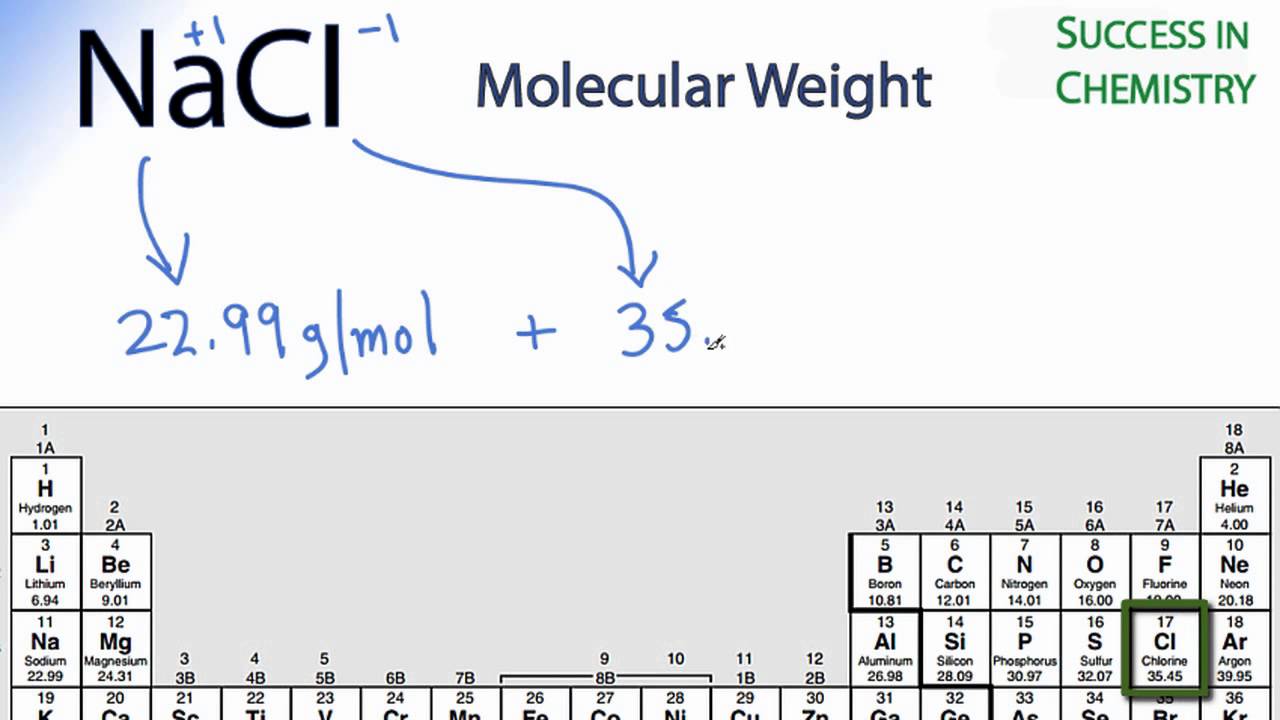
SOLVED: Molar mass of NaCl is 58.44 g/mol (this means, 1 mole of NaCl weighs 58.44 g). You are a researcher who needs to prepare several solutions. How many grams of NaCl
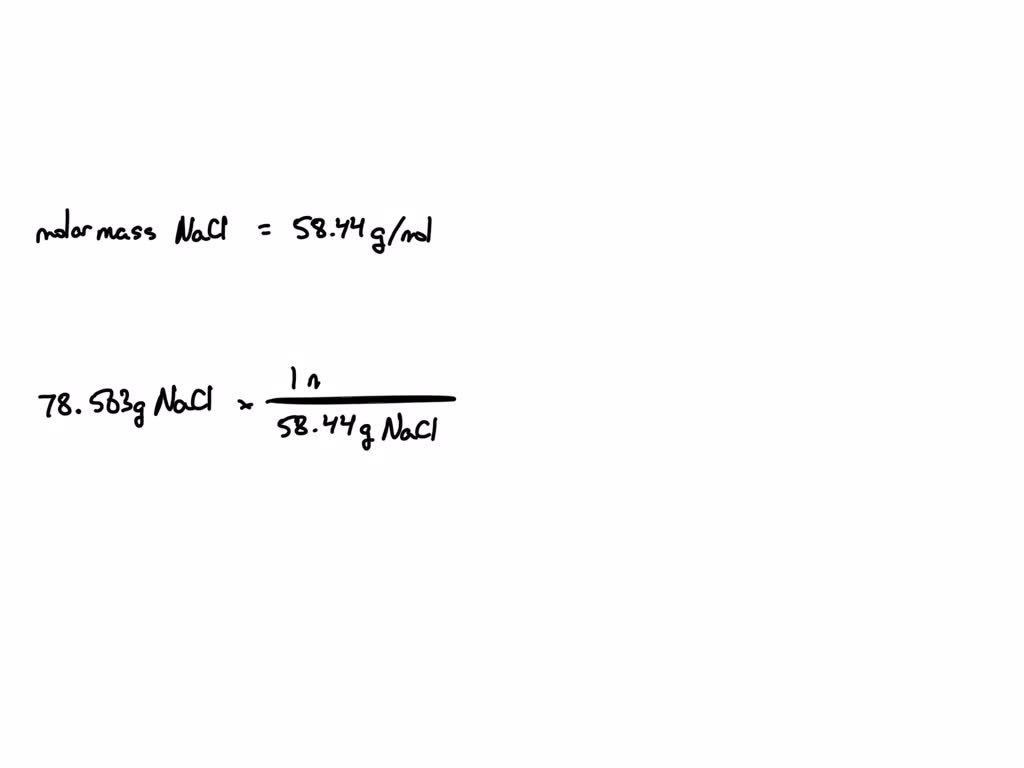
SOLVED: Calculate the number of moles (mol) of NaCl in 78.563 gNaCl, if there are 58.44 gNaCl per 1 mol NaCl. Report the answer using correct significant figures and units. Type answer:

The Mole & Chemical Quantities. The Mole Mole-the number of particles equal to the number of atoms in exactly 12.0 grams of carbon mol = 6.02 x. - ppt download

SOLUTION: Solutions and exercises in chemistry molarity mass calculations and empirical formulas - Studypool

Concentration Calculations Molarity. Objectives To calculate the molecular weight and moles of a substance To calculate the Molarity of a substance using. - ppt download

1. Determine the Gram Formula Mass of a compound 2. Convert between Grams and Moles 3. Convert between Moles and Grams. - ppt download